

A more precise definition of atmosphere, in terms of torr, is that there are exactly 760 torr in 1 atm. The unit millimeters of mercury is also called a torr, named after the Italian scientist Evangelista Torricelli, who invented the barometer in the mid-1600s. Other common units of pressure are the atmosphere (atm), which was originally defined as the average pressure of Earth’s atmosphere at sea level and mmHg (millimeters of mercury), which is the pressure generated by a column of mercury 1 mm high. A more useful unit of pressure is the bar, which is 100,000 Pa (1 bar = 100,000 Pa). One pascal is not a very large amount of pressure. This combined unit is redefined as a pascal (Pa). The basic unit of pressure is the newton per square meter (N/m 2). Pressure is what results when gas particles rebound off the walls of their container. Solids and liquids also experience and exert pressures, but under everyday conditions, pressure does not affect their properties. Due to the large amount of empty space between molecules in a gas, gases are compressible, so the pressure must be specified when other measurements are reported for gases. The force generated by gas particles divided by the area of the container walls yields pressure. There are forces involved as gas particles bounce off the container walls ( Figure 8.9 “Gas Pressure”). As gas particles are constantly moving, they are also constantly colliding with each other and with the walls of their container. 1 mmHg bng bao nhiu atm, gi tr chuyn i t 1mmHg ra atm nh th no, mmHg c gi y l milimet thy ngn, c ng dng nhiu trong vic o huyt p cho bnh nhn, ch s huyt p v cng quan trng bi mt ngi bnh thng ch s huyt p mc an ton l 100 n 120 mmHg, thp hoc. One of the statements of the kinetic theory mentions collisions. Thus, we can treat gas particles as tiny bits of matter whose identity isn’t important to certain physical properties. Although we know that there are, in fact, intermolecular interactions in real gases, the kinetic theory assumes that gas particles are so far apart that the individual particles don’t “feel” each other. The kinetic theory also states that there is no interaction between individual gas particles. However, the existence of real gases does not diminish the importance of the kinetic theory of gases.

Most gases show slight deviations from these statements and are called real gases. A gas that follows these statements perfectly is called an ideal gas. This means that all gases should behave similarly.
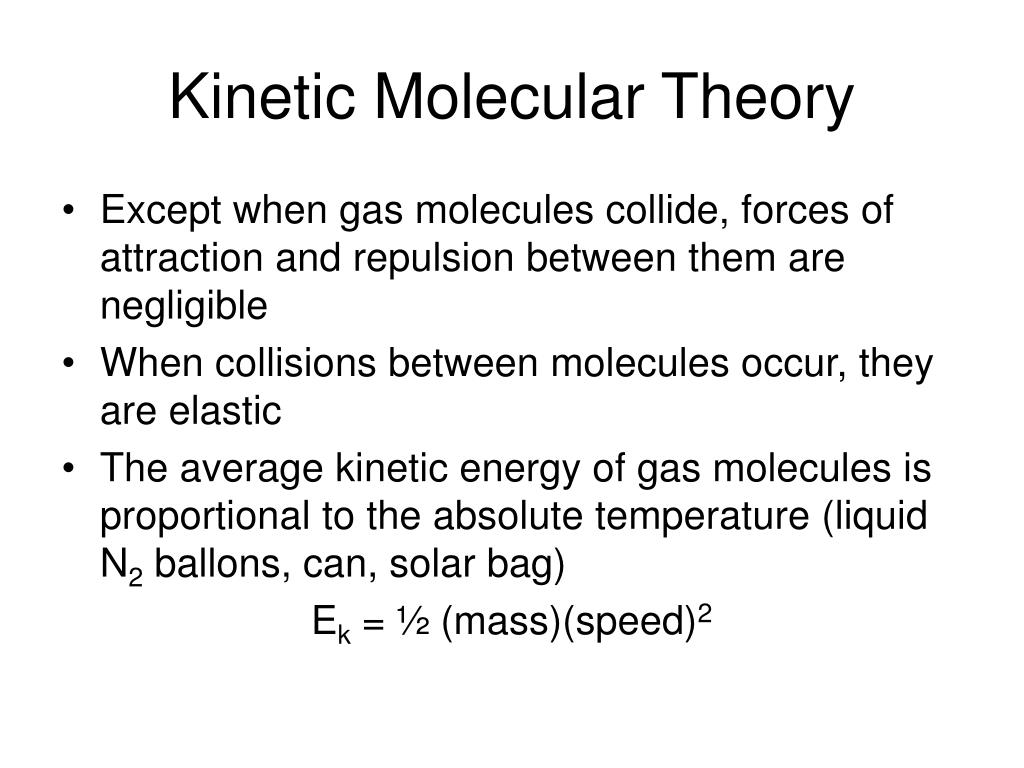
Notice that none of these statements relates to the identity of the gas.


 0 kommentar(er)
0 kommentar(er)
